0 引言
1 样品与实验方法
1.1 样品信息
表1 YC13-1-2井崖城组泥岩及对应干酪根样品基础地球化学参数Table 1 Geochemical parameters of mudstone and corresponding kerogen samples from Yacheng Formation of Well YC13-1-2 |
| 样品类型 | 深度/m | 层位 | 沉积相 | TOC /% | S 2/(mg/g) | I H /(mg/gTOC) | T max /ºC | R O/% | δ13Corg/‰ | 干酪根 类型 |
|---|---|---|---|---|---|---|---|---|---|---|
| 泥岩 | 3 989~4 014 | 崖城组 | 海陆过渡相 | 11.2 | 13.3 | 118.7 | 461 | 1.05 | -27.3 | III |
| 干酪根 | 3 989~4 014 | 崖城组 | 海陆过渡相 | 64.4 | 81.8 | 127.0 | 462 | - | -27.5 | III |
1.2 热模拟实验与气体分析
1.3 生烃动力学及甲烷碳同位素动力学参数
2 实验结果
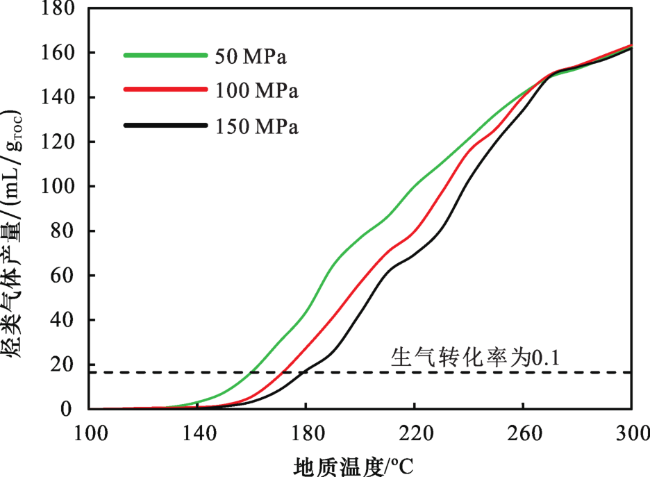
2.1 不同压力下烃类气体产量
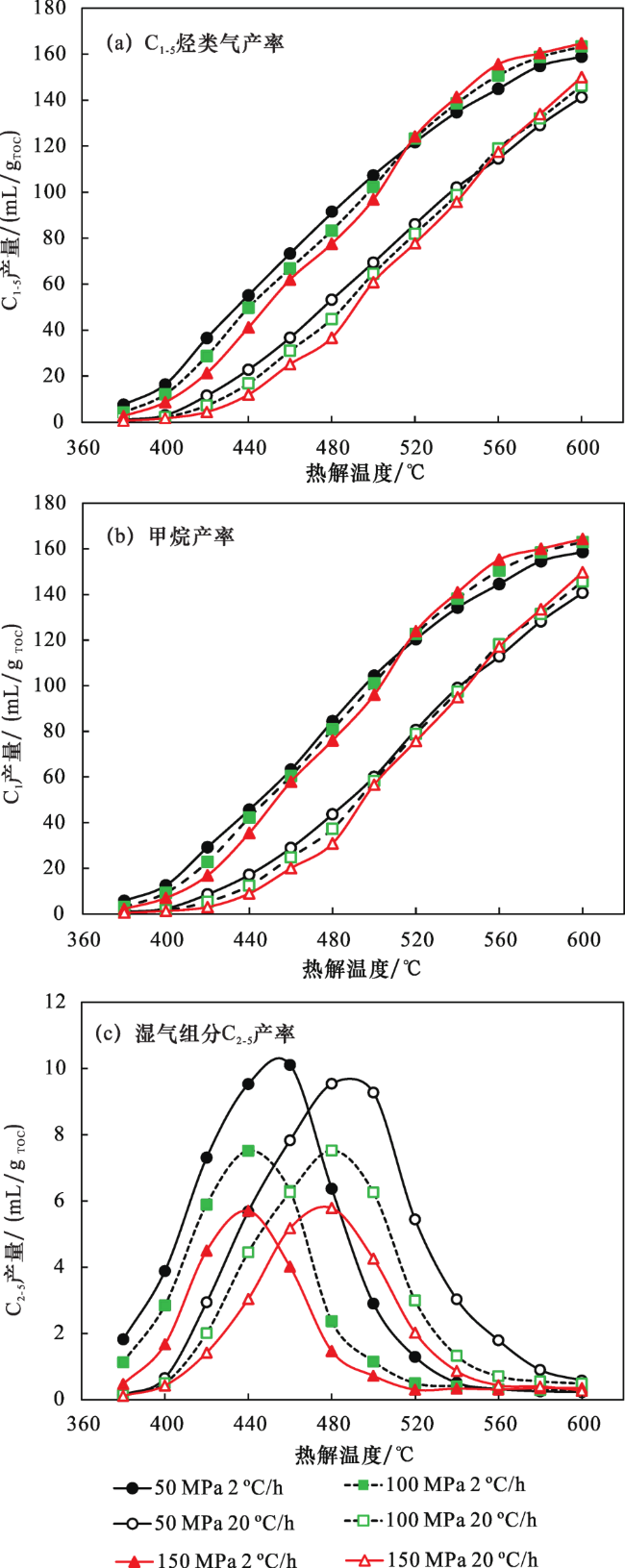
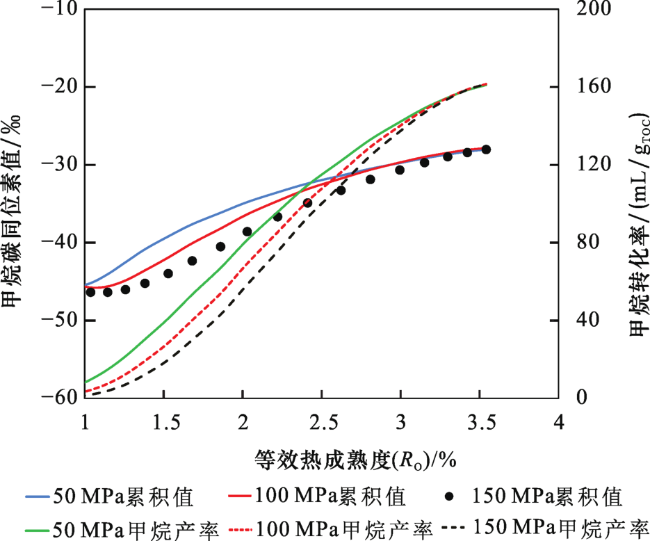
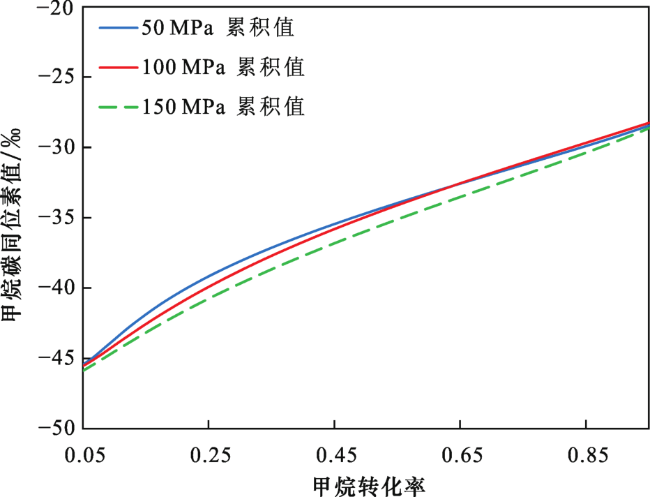
2.2 不同压力下甲烷碳同位素值
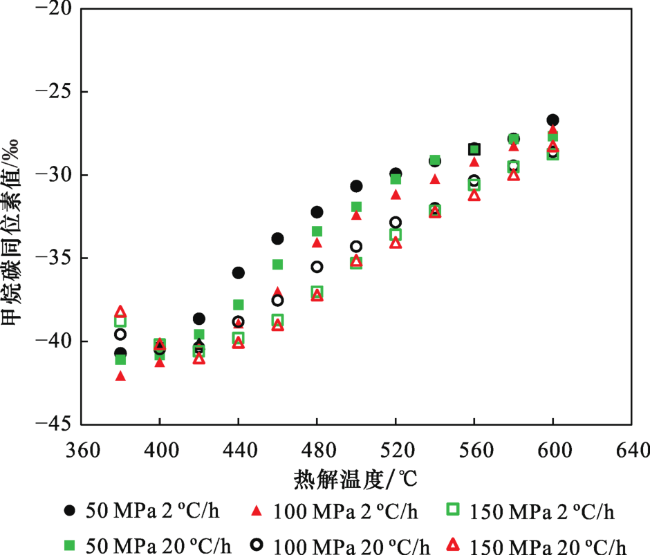
2.3 生烃动力学与甲烷碳同位素动力学参数拟合
表2 不同压力下YC13-1-2井崖城组干酪根生成甲烷碳同位素动力学参数Table 2 Various kinetic paramters of methane carbon isotope fraction for kerogen from Yacheng Formation of Well YC13-1-2 under different pressures |
| 压力/MPa | 13A/12A | βL/(cal/mol) | βH/(cal/mol) | μ/(kcal/mol) | Σ/% | δ13Cinit/‰ |
|---|---|---|---|---|---|---|
| 50 | 1.02 | 25 | 64 | 48.686 | 4.0 | -27.5 |
| 100 | 1.02 | 15 | 70 | 50.686 | 7.0 | -27.5 |
| 150 | 1.02 | 24 | 65 | 53.501 | 6.46 | -27.5 |
|


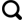
 甘公网安备 62010202000678号
甘公网安备 62010202000678号