0 引言
1 氮的海洋生物地球化学循环
2 氮同位素分馏与氮同位素组成的分布及其控制因素
2.1 海洋氮循环过程中的氮同位素分馏
2.1.1 固氮作用
表1 氮循环过程中发生的分馏情况(分馏因子ε≈δ15Nreact-δ15Nproduct) (据文献[22,45],修改)Table 1 Isotope fractionations in the process of nitrogen cycle (fractionation factor ε≈δ15Nreact-δ15Nproduct) (revised according to Refs.[22,45]) |
| 氮循环过程 | 同位素分馏范围/‰ | 保存情况 |
|---|---|---|
| 固氮作用 | Mo固氮酶 -2~+1 | 保存在氮受限的生态系统中的沉积物内 |
| Fe-V固氮酶 +6~+8 | ||
| 铵同化作用 | +14~+27 | 无记录 |
| 硝化作用 (NH4 +→NO2 -) | +14~+38 | 在化跃层进行完全,无保存 |
| 硝化作用 (NO2 -→NO3 -) | -12.8 | 在化跃层进行完全,无保存 |
| 硝酸盐同化作用 | +5~+10 | 在透光带进行完全,无保存 |
| 异化硝酸盐还原作用 | +30? | — |
| 反硝化作用 | +10~+30 | 通常在次氧带发生不完全反硝化,保存在同化NO3 -的生物体中 |
| 厌氧氨氧化作用 | +16或+23~+29 | 很难与反硝化区分开 |
2.1.2 再矿化作用
2.2.3 同化作用
2.1.4 硝化作用
2.1.5 异化硝酸盐还原作用(DNRA)
2.1.6 反硝化作用与厌氧氨氧化作用
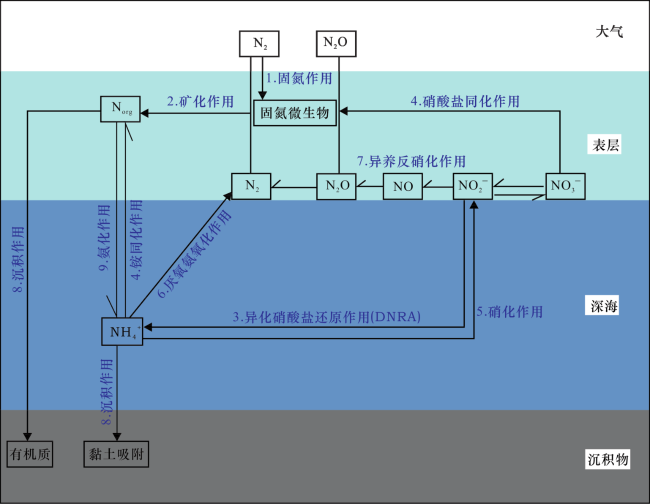
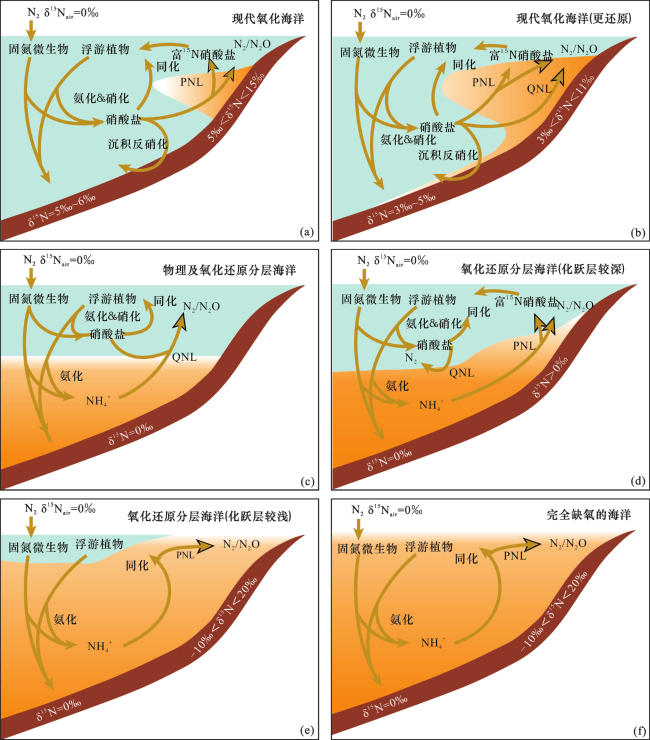
2.2 沉积岩中氮同位素组成的分布特征及其控制因素分析
2.2.1 偏负的δ15N值
表2 沉积物中氮同位素记录及可能的氮循环作用过程Table 2 Nitrogen isotope records in sediments and possible nitrogen cycling processes |
| δ15N分布 | 水体中可能占主导的N循环 | 沉积物δ15N值/‰ | 地区 |
|---|---|---|---|
| <-2‰ | 真光带缺氧区绿硫或紫硫 细菌同化作用 | 0~-8 | 现代黑海[60]、东地中海[61] |
| -4.7 | 华南三岔剖面寒武系牛蹄塘组[76] | ||
| 不完全铵同化作用 | <-2 | 印度古元古代晚期Aravalli Supergroup[59] | |
| ~-7.5 | 华南地区寒武系(2~3阶)[44] | ||
| -2‰~+1‰ | 蓝细菌固氮作用 | 0~+2 | 华南三岔剖面寒武系牛蹄塘组[76] |
| -2~+3.1,均值0±1 | 华南Chunye1井寒武系荷塘组[72] | ||
| ~-2 | Cariaco盆地[77] | ||
| -2~+1 | 黑海[64] | ||
| -1~+1 | 华南南盘江盆地太平剖面上二叠统—下三叠统[78] | ||
| >+2‰ | 不完全硝化作用 | +2~+18 | 白令海[66,67] |
| 不完全反硝化作用 | -2.6~+4.8,均值2.2±1.3 | 华南Chunye1井寒武系皮园村—荷塘组[72] | |
| +4~+9 | 华南毛石—中南剖面震旦系灯影组[73] | ||
| +4~+8 | 华南三峡地区震旦系陡山沱组[71] | ||
| +7.4 | Santa Barbara盆地[69] | ||
| >+11 | 阿拉伯海[70] | ||
| 硝酸盐同化、固氮及反硝化作用相平衡 | +1~+16,均值~+5 | 现代海洋沉积物[75] |


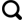
 甘公网安备 62010202000678号
甘公网安备 62010202000678号